They turn to Facebook to find a cure for diabetes— and became victims of Online Health Disinformation Exploiting Africans

By Olayinka Shehu
It began with a 2023 Facebook post claiming to cure diabetes; what was uncovered was a web of deception built on false hope.
No one responded meaningfully to Mantebe Raphoto’s plea for help. No other members of the group responded with concern or caution about the post that had appeared in a comment in a Facebook group.
“I need help,” she wrote in the comments section of a group that operates like a marketplace for health products claiming to cure diabetes.
She explained the adverse effect of the promoted product she had purchased from the group for her mother-in-law.
“I need help. I bought three bottles for my mother-in-law. After finishing the first bottle, her body started swelling. I don’t know what to do,” she pleaded.
The woman fromthe Southern African country of Lesotho had turned to the Facebook group that once promoted the product’s supposed curative properties for diabetes, now desperate and confused, after witnessing the troubling reaction her mother-in-law experienced from the pill she had bought online.
Far from the guidance or concern she had hoped for, the Facebook group administrator’s only response was a casual “okay.”
Raphoto made the comment three years ago. Since then, her Facebook account hasn’t been active. Calls and messages to her verified WhatsApp phone number, as well as attempts to contact her through Facebook Messenger, have been unsuccessful.
For those with diabetes and hypertension, the group acts as an unregulated marketplace and information exchange. Every day, it features dozens of health disinformation posts promoting cures, the majority of which lack verified approval, dosage recommendations, or evidence of safety.
Raphoto had purchased one of those alleged curative health products after being convinced by testimonials and bold promises. She wasn’t alone. Facebook groups like this one attract hundreds of members from all over Africa and beyond, drawn by the promise of hope and targeted by sellers who exploit illness for profit.
Raphoto’s experience is part of the evidence gathered over the last twenty months by disinformation and cyberthreats journalist Olayinka Shehu, who is investigating the tactics and actors behind the online sale of unregulated and unlicensed health products marketed as curative drugs to Africans.
The story begins on a bright afternoon in December 2023, when a casual stroll through Facebook leads to a suspicious advertisement promoting a so-called cure for diabetes. The advertisement used a digitally manipulated image, a deepfake video, and an AI-generated voice, all designed to convey credence to a curative product. A closer look at the Facebook page behind the advertising revealed a pattern: similar misleading strategies were being used to market a health product passed off as a curative product named GlucoPro, advertised as a diabetes cure. Further examination of the Meta Ad Library of the page uncovered a larger network of advertising promoting unverified cures for arthritis, hypertension, and high blood pressure, all of which used the same coordinated and misleading digital tactics.
Online research indicates that GlucoPro is a nutritional supplement, however unlicensed and unregulated, but in Facebook groups, it is being promoted as a diabetes treatment. Members of one group were advised to switch from approved diabetes drugs to GlucoPro.
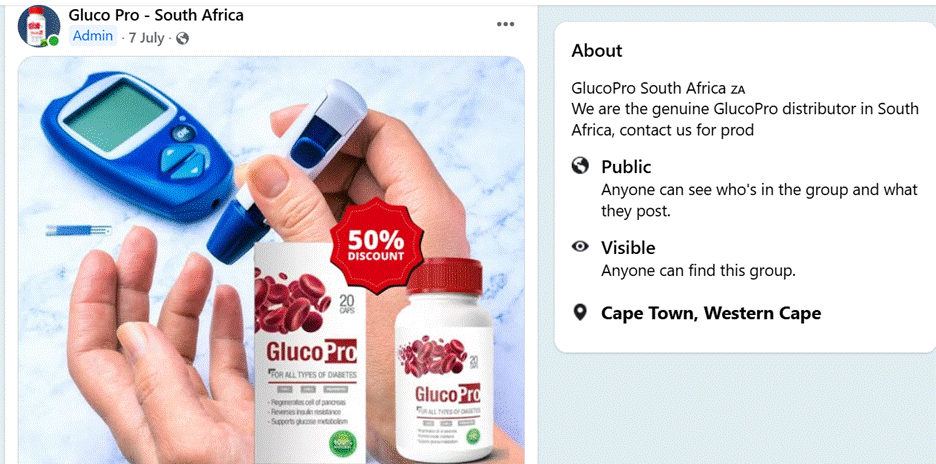
A screenshot of one of the GlucoPro groups on Facebook.
Dietary supplements are vitamins, minerals, and herbs that claim to improve health. According to the United States National Institutes of Health (NIH), a dietary supplement label may make claims about health benefits. However, unlike medications, supplements cannot claim to cure, treat, or prevent disease.
That year marked the first time GlucoPro surfaced on the journalist’s Facebook For You Page, but it wouldn’t be the last. Content promoting GlucoPro continued to appear as part of a recurring wave of fraudulent health adverts that took advantage of the platform’s algorithmic reach.
This investigation reveals how Facebook pages, Facebook groups, and websites that function as digital marketplaces that operate in plain sight with little to no oversight are used to sell GlucoPro and other supplements that are passed off as curative products and how Facebook has also turned into a silent enabler of a global trade in unreliable health products.
Diabetes is caused by dietary and lifestyle changes, as well as genetics, and once diagnosed, it can only be treated rather than cured.
In 2021, the World Health Organisation reported that 24 million persons in Africa had diabetes, with 13 million of those cases going undiagnosed. This represents the world’s largest percentage of undiagnosed diabetes cases. Although there is no known cure for diabetes, if you happen to be on the Facebook group, you might think that GlucoPro has been discovered as a cure.
Zee Seete, a woman from South Africa, believed this. She had fervently defended GlucoPro in June of 2024 when a fellow South African, Ntobeko Mvunyiswa, had posted a comment on the same group Raphoto had posted a comment in, labelling GlucoPro a scam after buying and using the alleged curative product and not seeing changes in her health.
According to Seete, her husband’s blood sugar levels dropped from 22.5 in April 2024 to 7.5 in June 2024. However, when contacted in July 2024 regarding the purported effectiveness of GlucoPro, she provided a different account, claiming that the product was not a cure for diabetes as claimed by an advertisement and characterising claims of its effectiveness as fraudulent.
Seete said her husband has type 2 diabetes and began using GlucoPro and the diabetes medication Synjardy together in February 2024. “For a month, we used GlucoPro with Synjardy, and I was gushing over how his blood sugar levels dropped. After a month he used GlucoPro alone. We didn’t check his blood sugar during that time, so after the capsules were completed, we tested to make sure it was still low. To our surprise, it was 19.5, which is extremely high.”
Seete’s introduction to GlucoPro came through an ad that appeared on her Facebook For You Page. She shared a screenshot of the advertisement offering GlucoPro as a diabetes cure that she said had shown up on her For You Page.
The GlucoPro promotion content ran on an account with the profile name Zandile Maseko. The account’s profile picture, however, was subjected to a Google Lens image-reverse search and found to belong to South African podiatrist Tshidi Malehlohonolo Mbonani, and not Zandile as alleged by the account. The name Zandile Maseko appears to be a common name among South Africans.
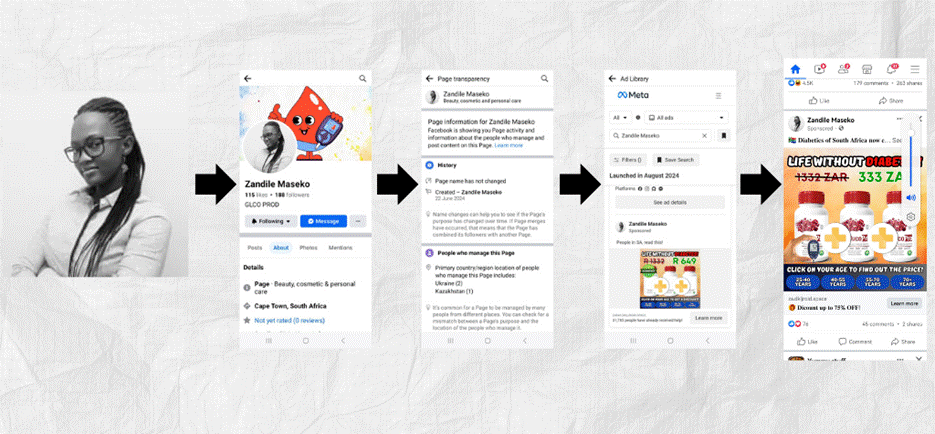
Collage of Facebook screenshots showing Tshidi Malehlohonolo Mbonani’s image being falsely presented as Zandile Maseko in a GlucoPro promotion.
Yamkela Kabese has the same story. She bought Glucopro for her husband who is battling diabetes after becoming aware of it online.
She said, “I paid R3,700 ($208 or N319,037) for a GlucoPro package that had 8 bottles of tablets, using my last money to buy those tablets for my husband. He used them for three months, and there was no change in his health.”
Who is behind the GlucoPro campaign and just how big is it?
It was discovered that the sale of GlucoPro and Cardioton, another supplement that was misrepresented as a curative product on Facebook, was not limited to South Africa and Nigeria. According to a Facebook analysis, people in six African countries, Nigeria, Tanzania, South Africa, Kenya, Zimbabwe and Lesotho purchased GlucoPro because they thought it would treat their diabetes.
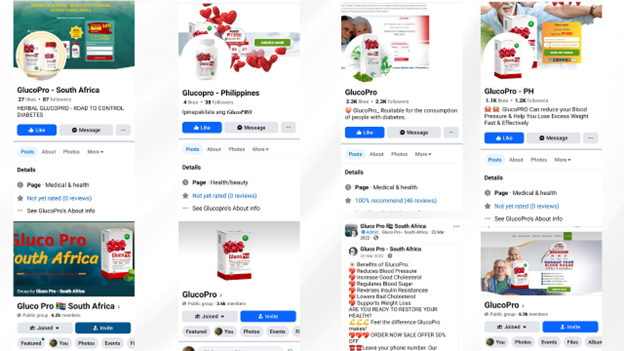
Screenshots of several Facebook pages and groups linked to GlucoPro promotion
The investigation also found that 152 Facebook pages and eleven groups with the name “GlucoPro” in their names exist on Facebook and promote the alleged diabetes cure in over ten countries worldwide, primarily in South America, Asia, and Africa.
Findings and details of GlucoPro operations which include the pages and groups have been shared with Meta (the company that owns Facebook).
Because the page profiles and groups are faceless and function as corporate accounts with no identifying individual or corporation associated with them, it is difficult to verify the validity of the materials they display. The GlucoPro campaign makes use of this loophole. By emulating legitimate firms’ promotion strategies in marketing and customer engagement and using Meta’s ad platform, it earns unjustified validity in the eyes of followers, many of whom believe GlucoPro effectively treats high blood pressure or diabetes.
Myanmar, one of the countries to which GlucoPro was promoted, issued a health warning to its citizens in January 2023, indicating that it is not a cure for diabetes.
The GlucoPro network creates country-specific pages and groups to further create the idea that it is locally based. Targeted Facebook users are more likely to believe content that seems to come from within their own groups, so this strategy helps it connect with them.
Under the surface of a local presence, however, there is a coordinated, cross-border activity taking place. A thorough analysis of more than 150 pages and page administrator locations reveals a pattern of activity in multiple countries. While the front-facing pages and group activities are tailored for audiences in Nigeria, South Africa, Philippines or Honduras, the back-end activities often refer to offshore operations, sometimes linked to affiliate marketing schemes or disinformation merchants. Furthermore, the network handlers seem to have developed a well-thought-out strategy that conceals the network’s financial objectives and reach, which are revealed by financial traces like ad spend. Digital evidence suggests that GlucoPro’s campaign intentionally combined aggressive monetisation with obscurity.
Glucopro-Honduras, one of the pages found during an analysis on Facebook in November 2024, contained an Indian registered phone number connected to a dormant Facebook account named Maria Beliaeva and a business WhatsApp account with the business name Nutratainment. After subjecting the account profile picture to Yandex reverse image search, it was discovered that the photograph belonged to a Russian person by the name of Nadezhda Kotkova.
The account appeared to have been used for the promotion of footwear on Facebook.
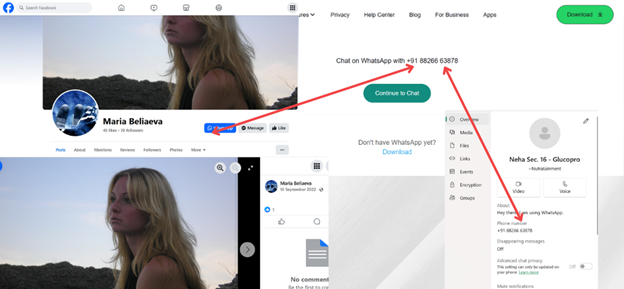
Screenshots of a dormant Facebook page connected to an Indian phone number associated with online GlucoPro promotion.
Additionally, the phone number was also subjected to Truecaller, a smartphone software that uses phone numbers to identify scammers and telemarketers. Truecaller showed that the phone number, had the name Nutratainment Spasmalir and was found to be an Indian registered phone number.
When Nutratainment Spasmalir was searched on Google, a Facebook page named Spasmalir-South Africa was the first result. Based on information gleaned from a Google search, Spasmalir is a gel that is particularly made to reduce joint pain, stop age-related degenerative changes, and treat radiculitis symptoms, including lumbosacral and cervical-thoracic types.
However, two Facebook reviews from previous customers criticise the products as fake and a waste of money.
Nutratainment was also not listed on the official sources for checking registered companies in South Africa, Nigeria, or India, three countries investigation shows operations extended to.
Searches were also conducted for exact or similar names such as Nutra-tainment, Nutratainment, and Nutritainment, as well as a manual lookup using the usual Indian Ministry of Corporate Affairs (MCA), which can be found at https://www.mca.gov.in, MCA search area. The search revealed that there are no active or inactive companies recorded in the MCA database with any version of the name.
The number was also discovered to be linked to another Facebook page named Volumin Guatemala, which has a Guatemalan location. Volumin is described as a natural tablet that helps to increase hearing and clean the ear canals.
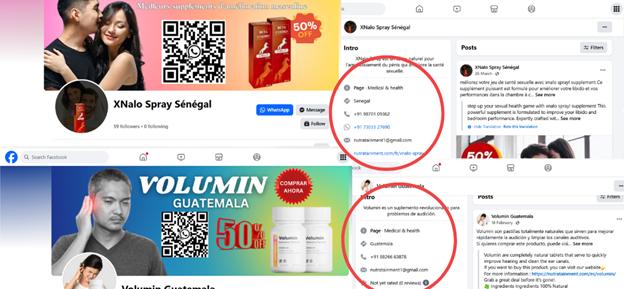
Collage of screenshots from the Facebook pages Volumin Guatemala and XNalo Spray, showing an Indian phone number linked to Nutratainment.
An examination of the page revealed an email address, nutratainment1@gmail.com, which, when searched alongside nutratainment@gmail.com another email linked to Nutratainment operations on Google and queried via AvatarAPI.com (an open-source tool for identifying names and profile pictures linked to email addresses), revealed that the email is registered under the name Sanket Nutra. Sanket is an Indian Hindi origin name and Nutra, a shortened form of the word nutraceutical–essentially short for dietary supplements that provide health benefits.
The email address, nutratainment1@gmail.com, was also found to be associated with another Facebook page, Sucrenorme-Cote d’Ivoire, which was last updated on March 3, 2025, and promotes Sucre Norme, which is said to be a potent ally in the fight against diabetes, and XNalo Spray, which is targeted at Senegal and is said to aid in sexual health. Two Indian-registered phone numbers are associated with both Facebook profiles.
The email address was also discovered on Letfindout, an online directory of Indian businesses, that shows that Nutratainment is headquartered in New Delhi, India.
Additionally, a search for Nutratainment online leads to an about.me page that describes Nutratainment as an online resource devoted to offering beneficial information regarding supplements for health and beauty.
The about.me, however, directs readers to click a URL that leads to a suspended Nutratainment website. The suspended website was subjected to Wayback Machine, an internet archiving platform that captures screenshots of websites and shows Nutratainment as an online platform that brands itself as a natural health supplement and promotes functional foods and supplements with health claims.
The website markets and sells Vigorense for male enhancement and prostate difficulties; Diafix, Diabextan, Glucoactive, Diabetic, and GlucoPro for diabetes; Hipanis for hypertension; and JointLab and Joint Cap for joint pain.
In 2021, the Republic of the Philippines Department of Health Food and Drug Administration advised against purchasing and using Vigorense and Diabextan.
While all of the products on the Nutratainment website appear to have been primarily targeted at Asian countries, with the exception of GlucoPro and Diabetic, which advert and sale have been seen targeted at Africans online.

A pack of Stop Diabetic product – Photo Credit: Health Element and Screenshot of Diabetic captured on b.Josefinaa.com
Stop Diabetic is described as a potent organic supplement designed to help patients efficiently control high blood sugar and diabetes. Stop Diabetic was observed in 2024 to be one of the products promoted by a Facebook page, Laut Products that promoted unlicensed and unregulated health related products in Nigeria.
One of the websites (screenshots) promoting Stop Diabetics used a stock photograph of a Black doctor uploaded in 2014 to pass it off as a Nigerian doctor named Dr. Ibrahim Abdullah, who created a medicine called “Stop Diabetics” as a permanent cure for diabetes.
The doctor, who was shown being interviewed, discouraged the use of metformin, a medicine used to treat high blood sugar levels, and instead advised readers to use Stop Diabetics, which was established in 2015 by Nigeria’s Institute of Endocrinology. However, the professional body focused on endocrinology in Nigeria is known as the Endocrine and Metabolism Society of Nigeria (EMSON), not the Institute of Endocrinology of Nigeria, as claimed by the website.
When contacted via WhatsApp about the cure claim attributed to Stop Diabetics, Hanisan Healthcare Private Limited, a research-based healthcare company engaged in contract manufacturing of pharmaceutical formulations, refuted the claim, stating that the product was manufactured as a supplement for diabetic patients rather than a drug.
Further investigation was also able to confirm that Nutratainment was linked to an Indian affiliate and digital marketing company, Traffury, through a phone number and address found on the Nutratainment website and on an Indian business directory website, PoweredIndia.
Screenshot from the Nutratainment website showing an address and phone number linked to Traffury.
Screenshot from CallupContact showing Traffury company details, and from PoweredIndia showing Nutratainment information.
Traffury and Nutratainment were also found to have the same contact person on PoweredIndia, Akash Gupta, who according to his LinkedIn profile was a previous director at Traffury and departed the company in 2024.
Traffury did not respond to an email inquiry requesting information about its link to Nutratainment, the Facebook pages and groups marketing GlucoPro and other health products online.
A check on PoweredIndia website three days after the email was sent shows that the Nutratainment page on the platform no longer exists.
Another health product found on Facebook, DiabeCode, said to fight against diabetes and promoted to Nigerians, was also linked to Nutratainment through Nutratainment1@gmail.com, and an Indian registered phone number found on the page.
Nutritainment appears to be a Traffury-operated platform that only exists on the internet, via its website, social media, and online directories, to promote unlicensed and regulated health products, including GlucoPro.
At the time of writing this report, it remains unclear whether Traffury was formally contracted to run the Nutratainment campaign involving GlucoPro and other unlicensed and unregulated health products or operated independently.
How do they operate?
The Facebook GlucoPro groups operate like an informal health marketplace. Seete, the South African woman, became a member after clicking on a Facebook ad that falsely promised a cure for diabetes. She is only one of many people who fall for deceptive ads that prey on vulnerable users.
GlucoPro South Africa was created in 2021 and has more than 6,000 members at the time of this report. The group frequently publishes posts endorsing unproven health products and purported natural remedies and often promotes them as substitutes for traditional medical care. These posts encourage false hope and discourage evidence-based treatment, creating a dangerous environment for people seeking real healthcare solutions.
To boost credibility, the group administrators and promoters frequently pretend to be satisfied customers or health professionals, posting testimonials and before-and-after images. They frequently refer prospective customers to WhatsApp or Facebook inboxes for private conversations, which are where the actual transactions occur. The group members are urged to buy these supplements passed off as curative drugs at exorbitant prices, with vague promises of curing diabetes or flushing out sugar from the body. There is no scientific evidence provided, and attempts to challenge the claim and complaints from past customers are usually ignored.
Two accounts Freni Khan and Amina Angel Patel operate on a GlucoPro Facebook group with more than six thousand members as sales representatives in South Africa. Both respond to anyone wanting information, as does the group administrator, who requests phone numbers in order to contact interested buyers.
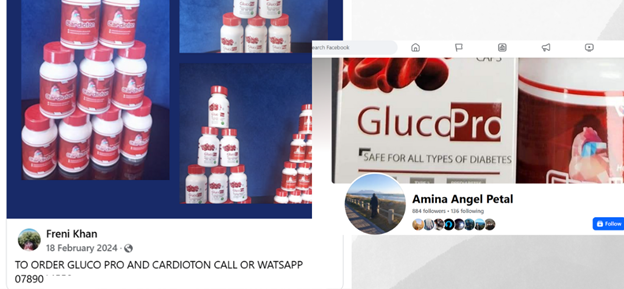
Collage image showing Freni Khan and Amina Petal promoting GlucoPro on Facebook.
A Facebook profile called GlucoPro – Philippines, which has over 4,100 followers, is the administrator of the GlucoPro Facebook group. The page and group both make unapproved medical claims under the about section, such as that GlucoPro “ends diabetes and gets rid of your diabetes forever.” It makes the false and scientifically unsupported claim that it can treat or cure both type 1 and type 2 diabetes.

Facebook group repurposed as a marketplace for GlucoPro promotions.
The product description also includes unverified health claims that are not supported by peer-reviewed research or regulatory approval. It claims to cut LDL cholesterol, control blood pressure, regulate blood sugar, reverse insulin resistance, and reduce obesity. The marketing language overuses superlatives, claiming that it is “better than any other product on the market” and a “never-before-seen revolutionary formula.”
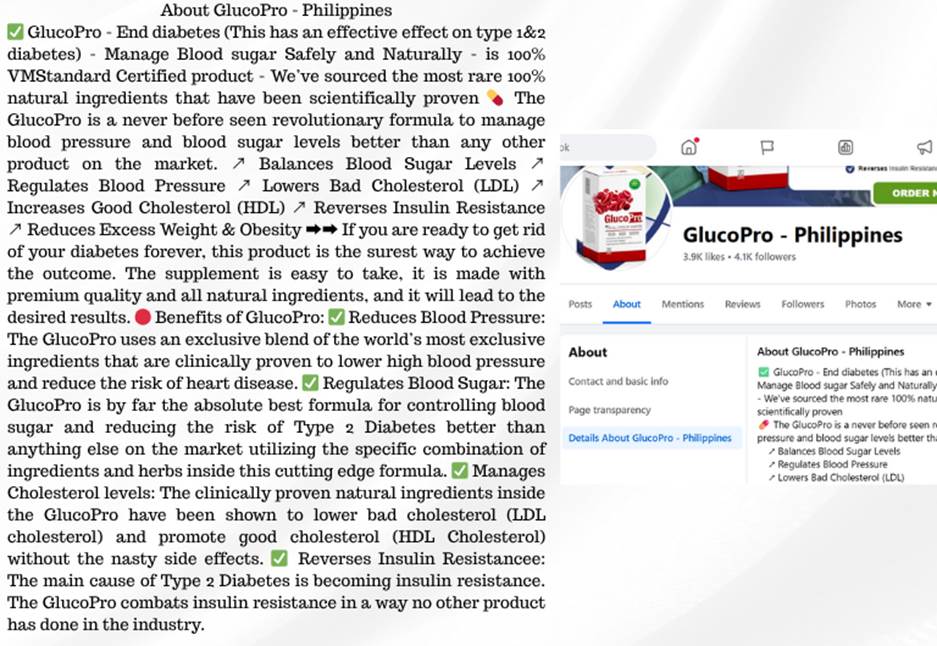
Screenshot from the About section of a GlucoPro Facebook page, alleging that GlucoPro cures diabetes.
The page makes questionable claims regarding certification and ingredients. While GlucoPro is advertised as “100% VMStandard Certified”, no credible medical or supplement regulatory body appears to have made this claim. Additionally, it claims that the product is “scientifically proven” without providing evidence from any health authorities or credible studies.
Amina Angel Patel did not respond when asked about her involvement in the GlucoPro sale in South Africa. However, when asked the same question, Freni Khan replied that she was only a sales agent and directed the reporter to contact the manufacturer. When requested for the manufacturer’s name and contact information, Khan refused to respond and subsequently blocked the reporter.
To further investigate the tactics observed in the group, a comment was posted on the GlucoPro South Africa Facebook group, indicating an interest in the product and expressing the need for it in Nigeria despite the group being intended for South Africans. A response was received within two hours, instructing the Journalist to drop his phone number for follow-up.
Three hours later, a WhatsApp message was sent to the phone number provided in the Facebook group. The message was sent by Sabeni Interconnect Calls Limited, a company with a WhatsApp business profile that claims to be the official distributor of dietary supplements for the prevention of various health conditions.
Sabeni Interconnect Calls Limited was registered in Nigeria in 2023, according to a search conducted on the Corporate Affairs Commission (CAC) website.
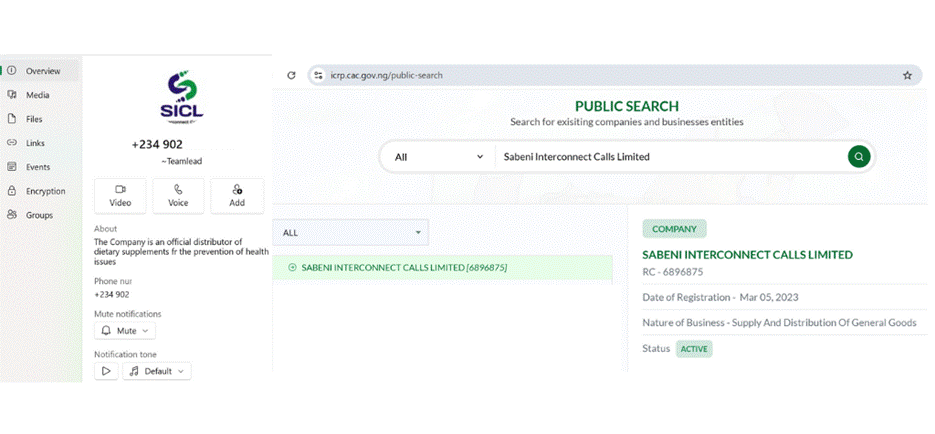
Screenshot of Sabeni Interconnect Calls Limited’s WhatsApp Business account and CAC search result.
The Journalist asked if GlucoPro could treat diabetes while pretending to be a prospective customer. The company in response on the WhatsApp business account affirms that GlucoPro has curative properties.
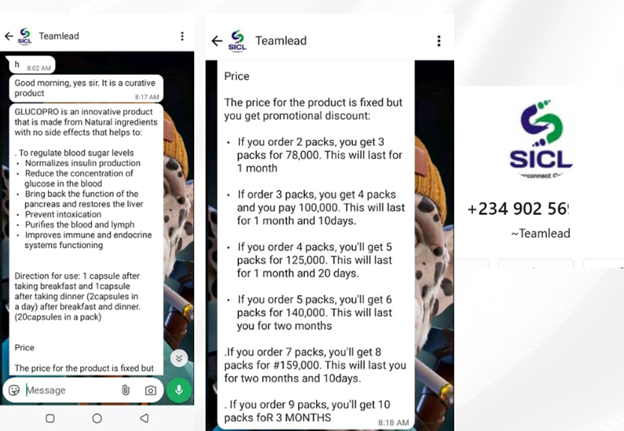
Screenshot of Sabeni Interconnect Calls Limited’s WhatsApp Business account claiming that GlucoPro has curative properties.
An inquiry was sent to Sabeni via its email address and the company’s WhatsApp business account, asking for information on why the company is marketing GlucoPro for sale in Nigeria despite the product not having been approved by any regulatory authority in the country. The email also questioned the company’s claims that the product could cure diabetes, given that no national or international health agency had certified it as such and it links to the GlucoPro Facebook group and Nutratainment. The company has not responded to the email as of the time of publication.
More consumers speak
Thulani Zengele, a staunch South African follower of alternative medicine, said that after taking GlucoPro for 60 days, his diabetes was healed. “My doctor confirmed that my glucose is normal, and I didn’t take any chronic medications,” he stated. Zengele, who is presently using another product, CardioZoom, for hypertension, completely rejects conventional medications. He accuses what he calls “racist medical boards” of suppressing African medicines to promote Western drugs, which he claims are profit-driven. “My parents died taking your so-called approved medicines,” he said in an interview conducted via WhatsApp. “Who approved of our forefathers’ use of herbs to improve their quality of life? Nobody.” Yet it worked.”
Zengele’s ideas are strongly influenced by what he sees online. “I found these healthcare professionals on Facebook”, he said. Insisting that GlucoPro had curative properties. His views reflect a broader disinformation trend on Facebook, where videos and posts promote unlicensed health products as cures for chronic conditions like diabetes, high blood pressure, arthritis, and joint pain. Zengele repeated similar claims, stating that pharmaceutical companies “kill us in numbers and promote dependence” for profit.
These videos frequently make unproven assertions that pharmaceutical corporations are seeking to prevent GlucoPro’s distribution, which Zengele repeats. He blames these companies for his personal losses, claiming they are the reason he turned to unlicensed products such as GlucoPro and CardioZoom. He refused to accept that the investigation has shown that the two products are dietary supplements passed off as curative health products and not drugs with curative properties.
He also assumed that GlucoPro was a South African product; however, when informed by the journalist that GlucoPro did not originate in South Africa, Zengele responded angrily, accusing the journalist of bias. “You speak for the pharmaceutical companies. You already exhibit a preference for drugs rejected by racist medical bodies that oppose African values and customs.”
Unlike Zengele, another South African, Donald Dorasamy, said GlucoPro didn’t work for him. Dorasamy has type 2 diabetes and spent R1,200 (₦105,737 or $65.79) on two bottles after being misled by an ad claiming it could cure diabetes in a week. “I’m still on my chronic meds,” he said. “I realised they’re just trying to make their product another long-term supplement, not a real cure.”
It was also discovered that GlucoPro was available for purchase on e-commerce sites Jumia and Jiji as a health product for diabetes and hypertension. Misleading health claims, unverified medical promises, and scientifically unsupported claims are used to describe the product on both platforms, which could put customers at risk, especially those who are treating diabetes or prediabetes.
It is advertised on Jumia as a product that can prevent heart disease, diabetes, and cancers. It also states under the product detail that it’s produced in India. GlucoPro is alleged to normalise psycho-emotional states, regulates immune function, restores metabolism, and treats or cures chronic conditions, including diabetes and hypertension. However, checks show no clinical research and government bodies have validated or approved any of these claims.There is also no evidence to show that GlucoPro is approved by the Indian Central Drugs Standard Control Organisation (CDSCO) or by the Food Safety and Standards Authority of India (FSSAI).
GlucoPro on Jiji is said to be approved by the U.S. Food and Drug Administration (FDA). However, a review of the official FDA database reveals that no such product is registered or permitted.
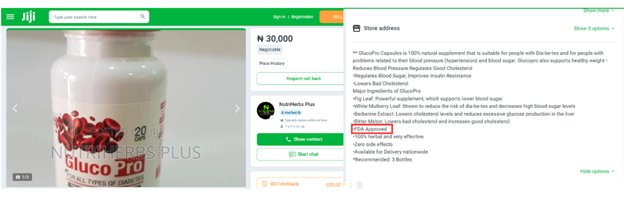
Screenshot of a GlucoPro listing on the e-commerce platform Jiji, claiming it is approved by the FDA.
It also alleged that the product has no negative effects and promoted it as effective in controlling weight, blood pressure, cholesterol, and insulin resistance. However, the seller did not post any evidence on Jiji to support these claims about GlucoPro.
NutriHerbs Plus is the Jiji seller for GlucoPro. The vendor information section states that they only sell NAFDAC-approved products. However, NAFDAC has not registered GlucoPro.
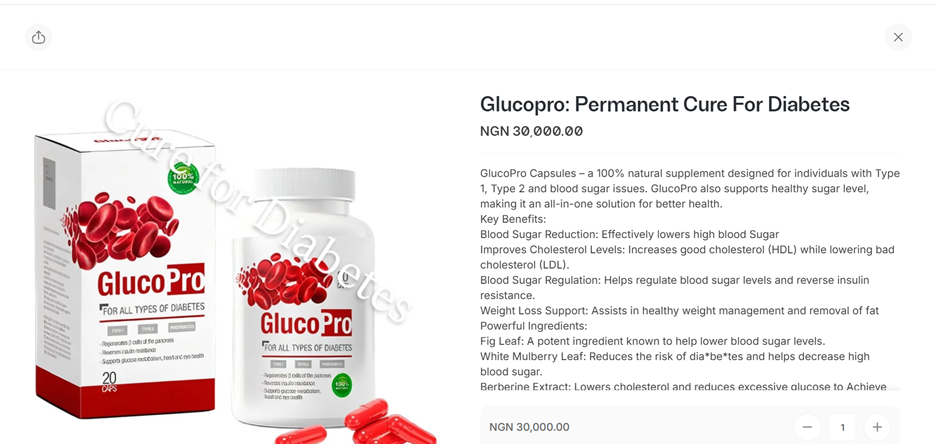
Screenshot from Nutriherbs Plus website claiming that GlucoPro is a permanent cure for diabetes.
Further examination of the NutriHerbs Plus website shows it is involved in the promotion and sale of unlicensed and unregulated health products. On the website, it describes GlucoPro as a permanent cure for diabetes.
NutriHerbs did not respond to an email and WhatsApp inquiry requesting information about GlucoPro’s registration and evidence supporting its curative claims.
Notwithstanding these warning signs, GlucoPro and other health products with purportedly curative health benefits are nonetheless sold on Jumia and Jiji’s platforms without NAFDAC clearance. At the time of writing this story, neither company responded to requests for comment on their policy and vetting procedures for unregulated and unlicensed health products promotional content published on their platforms.
Meta, Google Ads, fuel unlicensed Health Products campaigns
A Nigerian-born, US-based professor and chair of the Department of Biomedical Engineering at the University of Texas Southwestern Medical Centre, Samuel Achilefu has become an online sensation, expert, and pioneer of new medicine. His image and footage have been used in dozens of video advertisements that promote treatments for conditions like diabetes, cancer, high blood pressure, and arthritis.
However, most people who comment, like, share, and take action on the promoted content URL that features Professor Achilefu’s photographs and videos are unaware that it has been digitally manipulated. His voice had been cloned to fit the videos, giving the impression that he has pioneered or is endorsing a revolutionary medicine with potential cures for numerous ailments.
However this wasn’t just a digital content or website meant to discredit Achilefu and his achievements, they use his voice, image and video for their benefit.
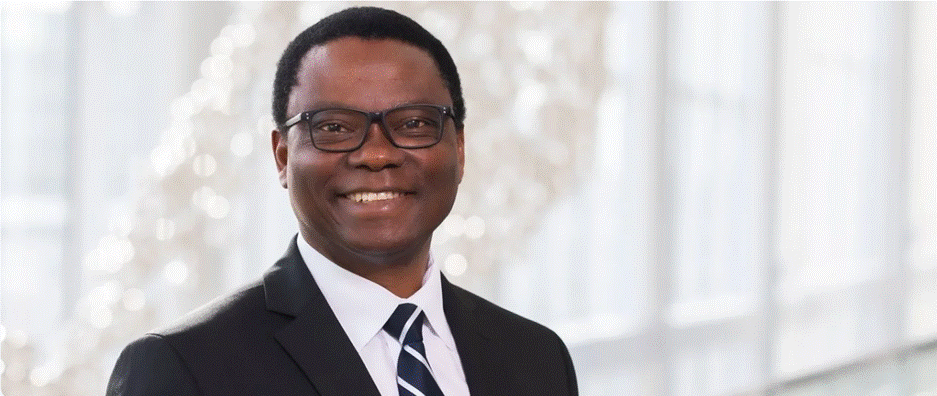
Photo of Dr. Samuel Achilefu
He has appeared on content and websites promoting GlucoPro, Cardioton, Cordix, Cardizoom, etc. as curative health products for different types of diseases.
Dr. Achilefu, in response to one of the videos on his LinkedIn account, denied endorsing any of those products.
Fact-checking analysis was conducted on over fifty websites and videos, some of which included Achilefu. It showed that the videos and website content were part of a global scam that involved selling people in several countries unlicensed and unregulated health products alleged to have curative properties. To operate the scam, the scammers create fake websites using logos and features belonging to several media companies, including Al Jazeera, CNN, BBC, NBC, Vanguard Nigeria, NTA, The Nation, Channels Television, TV Continental, and Arise TV.
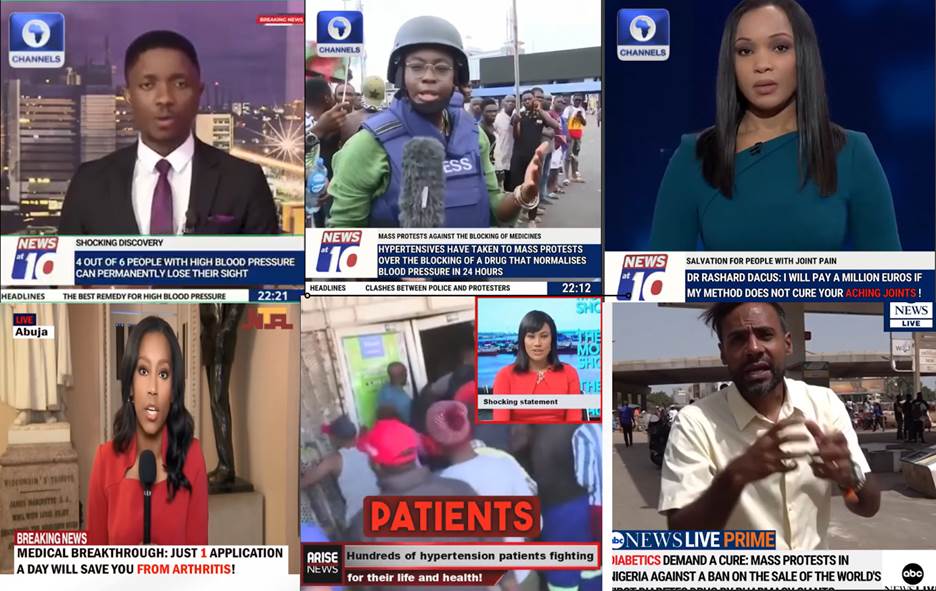
Screenshots of some broadcasters found on digitally manipulated videos on Facebook
Some of the pages design their websites to look like popular media platforms.
Impersonating popular and genuine media platforms is to portray the content as credible. Click to view some of the fake websites promoting unlicensed health products.
A Nigerian, Sunday Asuquo Eyoh, who used GlucoPro, expressed his dissatisfaction in the comment section of a promotional post published by the Fuerza Interior Facebook page. He wrote, “This product is not working. I’ve taken six bottles, and it still doesn’t work.”
Since 2023, social media monitoring has revealed dozens of Facebook advertisements that contain inaccurate information about at least twenty well-known practitioners, including professionals in medical fields and media personalities like reporters, presenters, and broadcasters. These advertisements all direct viewers to fake news articles with identical headlines promoting unlicensed curative health products.
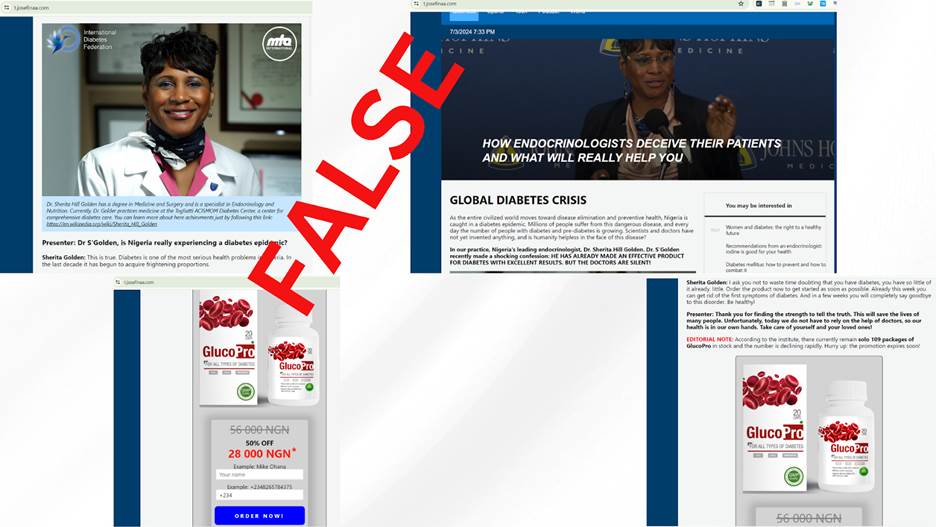
Screenshots from an alleged interview with Professor Sherita Hill Golden published by one of the website marketing GlucoPro.
The strategy observed deployed by those in charge of the websites and promotions involved three stages in their operations: Meta ads lead victims to fake news websites with articles featuring public figures and with details about a new drug and its maker. The next stage is the transaction stage.
These ads continue to thrive violating Meta’s advertising standards on domain impersonation, spam, and fraud-related policies, among others.
GlucoPro and other promoted solutions are surrounded by a web of false, misleading, and potentially dangerous claims that target people in distress. People who are under stress from a chronic illness or who have recently received a diagnosis are more vulnerable. In their search for solutions, they are led into algorithm-driven echo chambers that make exaggerated and false health claims.
Facebook’s recommendation system sends desperate people to dramatic videos and testimonials that promote unlicensed alleged curative health products for diseases, including high blood pressure, diabetes, arthritis, and joint pain. These ads usually combine conspiracy theories, such as the notion that Nigerian pharmaceutical corporations are hiding a diabetes medication, with fake success stories and pseudoscience. A recurring theme is that drugstore chains don’t stock these curative health products not because of safety issues but rather because they are profit-driven and don’t care about patient welfare. These emotionally charged narratives attempt to legitimise dubious products and erode public trust in medical institutions by inciting distrust and anger.
The links on some of the videos lead to websites with a wheel of fortune where the winner of some lottery-type prize will receive a 50% discount on that day only. Visitors still have a chance to win even if they visit the website the following day, the day after, or on any other day. All they do is give their name and phone number so that someone can get in touch with them.
An OSINT and Disinformation researcher, Kunle Adebajo, said the videos seemed to work quite well in the promotion of uncertified medical solutions, which not only deprived people of their hard-earned money but also endangered their lives.
“These actors would often use the images of newsreaders or other reputable individuals. And they even promote some of these posts, meaning the content moderation and ad review mechanisms are very weak. Social media platforms need to do more to track such harmful content. They should empower users to easily report them and bolster their capacity to swiftly work on such complaints,” Adebajo said.
In one instance, a page named Dr Emily Johnson, whose profile image was a stock photo that was posted on iStock in 2016, published a video that featured Professor Samuel Achilefu. One video stated that Dr Achilefu had discovered a 99% effective therapy for hypertension. The video was digitally manipulated, and the clone voice of the Arise TV logo and presenter Ojy Okpe was used. In a different video, he supposedly discussed the African hypertension epidemic and promoted a natural remedy that is supposed to bring blood pressure back to normal in a few short weeks. Both videos promoted Normatone and Cardiovax by linking to a fake website that used the Arise TV logo.
An Abuja, Nigeria–based doctor, Kingsley Onwuka, said, “Hypertension is a lifelong condition that can only be well-controlled with lifestyle modification and/or drugs. No one is said to be cured per se.”
Onwuka added, “Whether arthritis is curable depends on the type of arthritis and the age of the patient. As a doctor, I don’t prescribe herbal supplements because their contents, dosages, and side effects have not been proven. They have not undergone clinical trials.”
Similarly, a Facebook page purportedly belonging to a medical doctor, Dr Matthew Lee, featured Arise TV presenter Ojy Okpe and the CNN logo in a sponsored article about diabetes set against the backdrop of Arise TV. It promoted a 98% effective blood pressure-lowering medicine and sent users to a link for purchases. However, a Google reverse image search revealed that the profile picture, purportedly of Dr Lee, was actually that of Dr Robert Kruger, a physician in the U.S. Air Force. A Meta Ad Library analysis of the page revealed that 16 posts, 15 in Arabic and one in English that were intended for Nigerian users, were published in June 2024.
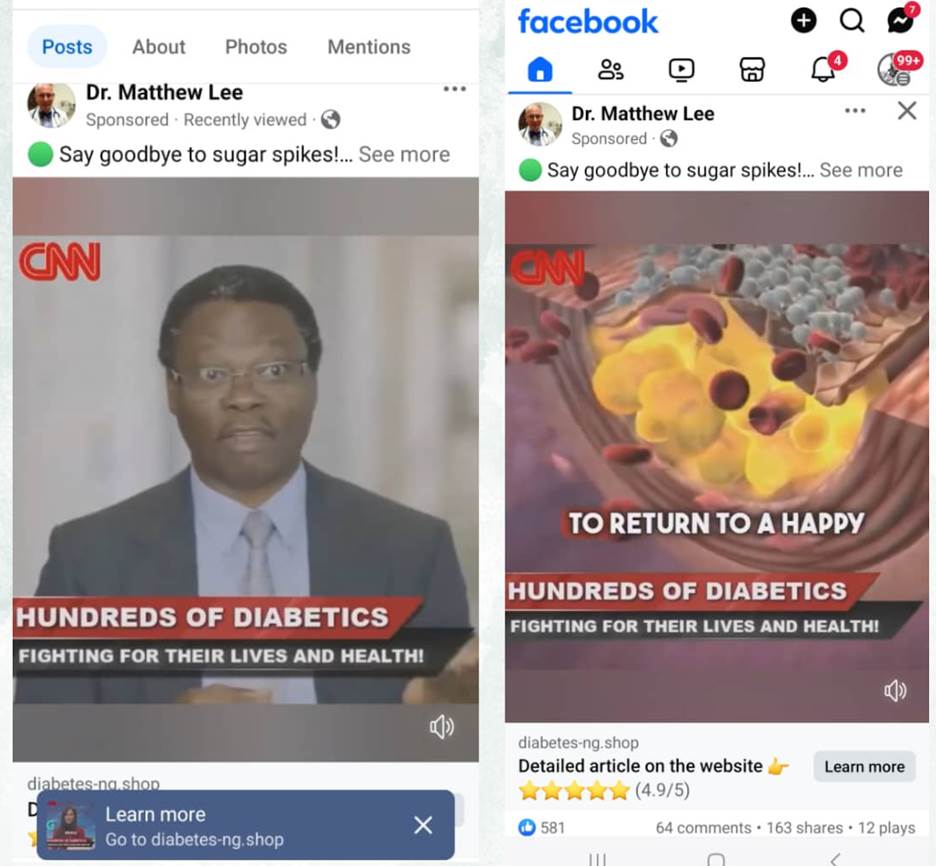
Screenshots from Matthew Lee’s page
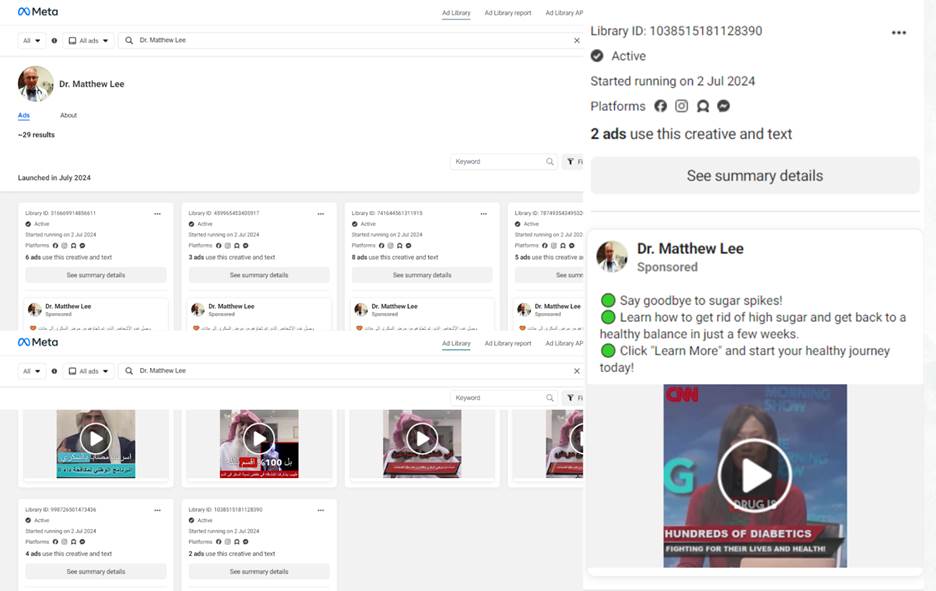
Meta-ad library of Dr. Matthew Lee’s page
Anwar Rida (أنور رضا), another page, used Achilefu’s name and image in three video adverts. Cordis, Normatone, and Cardiovax advertised that a single course of treatment could cure cardiovascular diseases and restore blood pressure to normal levels. PT SJA Global Cosmindo, an Indonesian company, was designated the producer on a Cordis label. When contacted via WhatsApp, the company denied making the product, claiming that it only manufactures cosmetics, not pharmaceuticals.
In another example, a page called Natural Media shared a video in June 2024 purporting to show Amaka Udeh, a former Arise TV programme host, interviewing Dr Chikwe Ihekweazu, the former director-general of the NCDC. The video took viewers to a fake website that promoted Cardizoom and made up the story that he was fired for finding a way to treat hypertension. Actually, Dr Ihekweazu quit his job willingly in 2021 to work for the World Health Organisation. Unsynchronised lip movements and facial distortion were among the obvious indications of AI manipulation in the video.
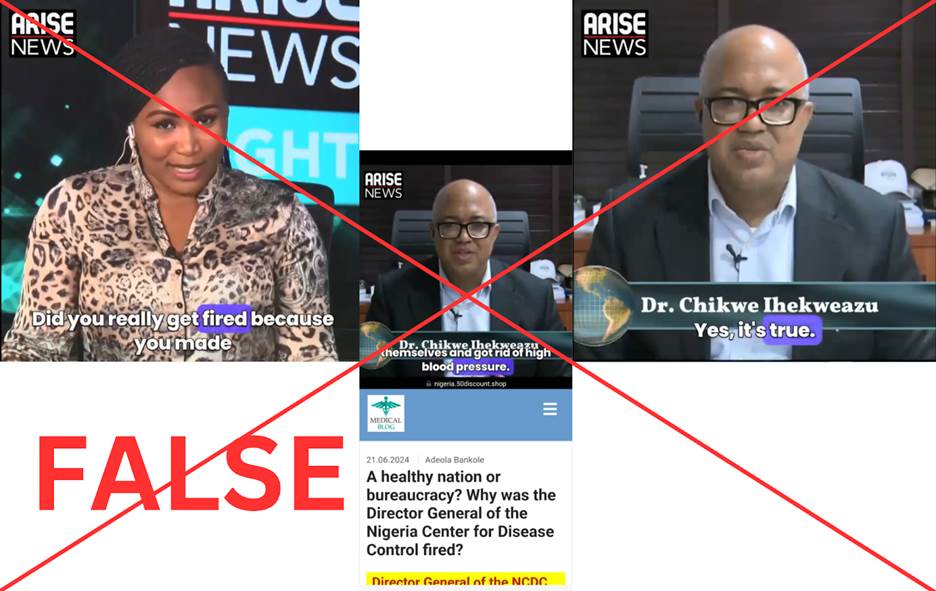
Screenshots from video uploaded by Natural Media page
A Nigerian doctor and influencer, Egemba Chinoso Fidelis, also known as Aproko Doctor, publicly denied endorsing Cardizoom on March 25, 2025, in a Facebook statement, following an AI-manipulated video allegedly showing him recommending the drug for high blood pressure and warning against prescribed drugs such as metoprolol, lisinopril, and amlodipine.
Another manipulated video featured Nobel laureate Professor Wole Soyinka with a voiceover claiming to have suffered high blood pressure for more than 50 years. Viewers were directed to a fake website that advertised Normatone as a treatment and had the Legit News logo. The original Soyinka video used in the promoted content was found via a reverse image search on a Louisiana Channel YouTube video from December 2023.

Screenshot from altered video of Professor Wole Soyinka found on Facebook
These are just a few of hundreds of AI-generated videos spreading through Meta ads to target a wider audience with false health claims. Over 50 pages were found to have used the marketing tactic of employing digitally manipulated artificial intelligence (AI) videos and images for promoting unlicensed health products on Meta-owned Facebook and Instagram between November 2023 and July 2025.
Facebook pages observed promoting unlicensed health products with alleged curative properties include The Walking Tall, Family Tree, Dr. Matthew Lee, Fluate, Dr. Emily Johnson, A Medical Mistery, Anwar Rida, Deal Moth 31, Midhawi, Suhileatan Health Good, and Natural Media. Additional pages and their content can be viewed here.
Meta did not respond to two detailed email questions submitted to its media email account, one on June 27, 2024, and the other on August 11, 2025. The emails contained names and information for Facebook pages that used digitally manipulated and AI-generated content to market unregulated and unlicensed health products for high blood pressure, diabetes, arthritis, joint pain, and low sexual performance. These pages used Meta Ads Manager to target a wider audience.
While Meta platforms (Facebook and Instagram) appear to be the primary channels used to promote these medications, it was also observed that the actors deployed Google Ads as part of their broader digital marketing strategy to reach a wider audience. A search on the Google Ads Transparency Centre revealed that products such as GlucoPro, Insulux, Xenorpost, Spasmair, Fast Active, Cardioton, and Metabon have been actively promoted through Google Ads targeting users in Nigeria, Tanzania, Cameroon, and South Africa.
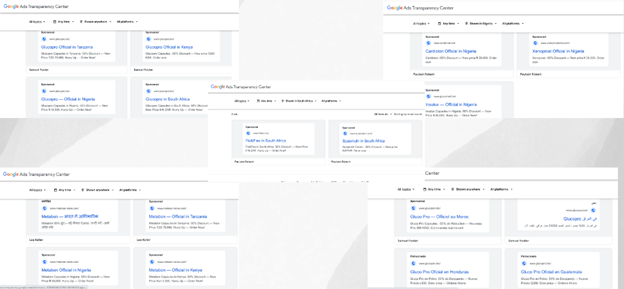
Screenshots of GlucoPro and other health product ads on Google, captured from the Google Ads Transparency Center.
Four names, Alicia Freeman, Samuel Foster, Lea Keller, and Paulson Robert, appear on the Google Ads Transparency Centre as sponsors of these ads across multiple countries. According to the platform, Samuel Foster is listed as based in the United Kingdom, Paulson Robert in Poland, Lee Keller in Germany, and Alicia Freeman in South Africa. However, online searches yielded no verifiable information to confirm the identities or professional affiliations of any of these individuals, raising concerns that these names may be fabricated or used as proxies.
The ads use a deceptive marketing structure, with websites like glucopro.bio deploying subdirectories such as /ng/ to target Nigerian audiences specifically. This indicates region-specific content rotation, a common tactic in global fraud schemes. The URL parameters, such as utm_source=google, utm_term=gluco pro, and gclid, confirm that these are paid search campaigns, making the product more visible to users actively searching for diabetes treatments.
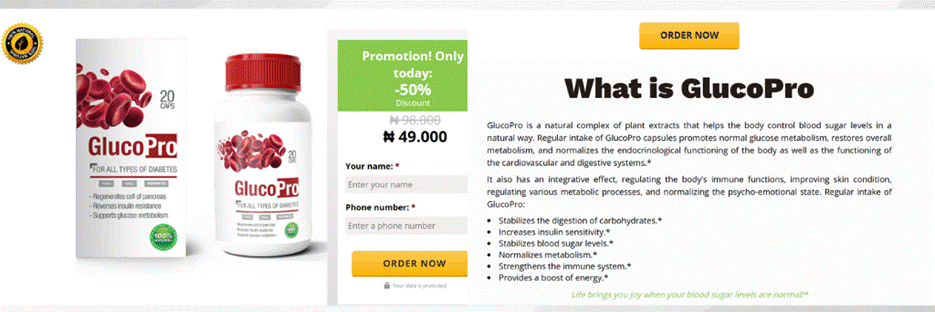
Screenshots from the GlucoPro website
The landing pages themselves are filled with false and dangerous medical claims, including “Helps cure type 2 diabetes naturally”, “Stabilises blood sugar after the first week”, “Recommended by doctors”, “Restores pancreatic function”, and “No side effects”. These claims are not supported by any clinical evidence or regulatory approval and are misleading to vulnerable users. Similar messaging was observed in ads targeting users in South Africa and Tanzania, with unregulated supplements being falsely marketed as cures for chronic illnesses.
Two Facebook pages, 40 subdomains – How the story began
Products like GlucoPro have been promoted on Facebook since before December 2023, when the journalist first became aware of it through a promotion by the Facebook page Laut Product. This page wasn’t functioning alone; it co-existed with another page called Eco Puzzling, and the two pages used Facebook advertisements to market a variety of unproven remedies for long-term ailments like diabetes, high blood pressure, arthritis, and joint pain.
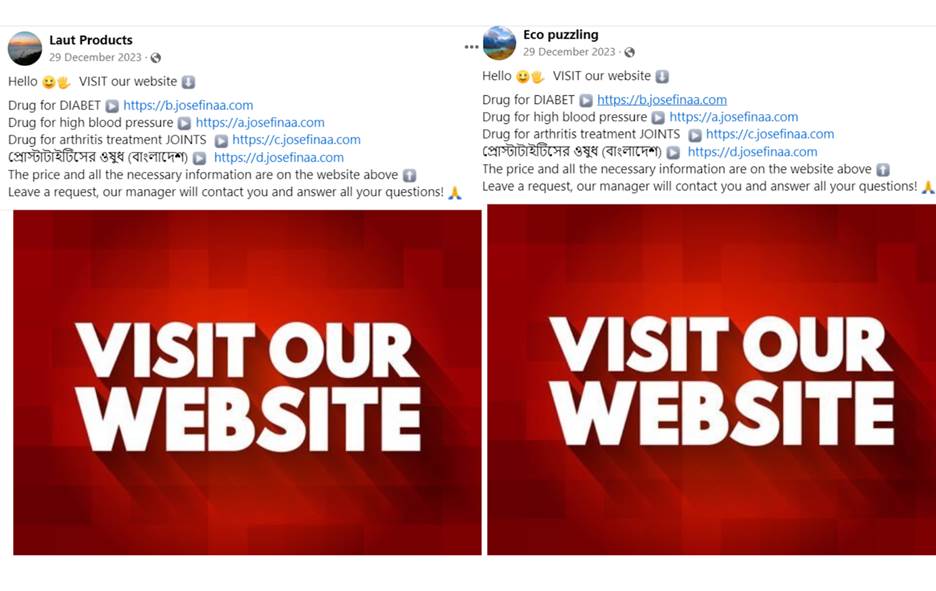
Screenshots of Laut Products and Eco Puzzling post on December 29, 2023.
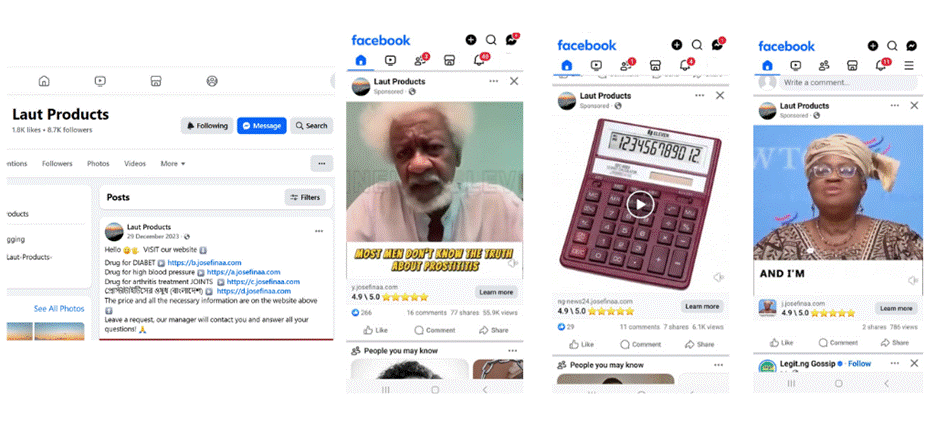
Screenshots from Laut Products’ Facebook page showcasing the content they promoted.
To gain deeper insight into their operations, the timelines and ad libraries for both pages were examined. A combination of paid and open-source intelligence (OSINT) tools was used to examine the website URLs posted on the pages. These tools made it possible to identify patterns, examine web infrastructure, and gain a better understanding of the overall plan behind fraudulent advertising campaigns.
While the Eco Puzzling ad library was banned after running a promotion targeting European countries, Austria and Germany, Laut Product continues to run promotions targeting countries, mainly Nigeria and India.
Both pages were observed to have the same post published on December 29, 2023, on their timelines calling for website visits for curative health products for diabetics, blood pressure, arthritis, and prostatitis, all written in English, and another in Bangla, the official language of Bangladesh. The videos that went with the posts generated thousands of views and received hundreds of comments from those who wanted to know how to buy the health product with the supposed curative properties .
The two pages used the strategy of not allowing their videos to appear on their timelines, as they were uploaded through the Facebook ad manager and promoted. The URL links appearing on the posts served as the landing page for the sponsored content. Through this method, products were sold while public scrutiny was avoided. This strategy was subsequently observed on several other Facebook pages that featured similar unlicensed health products, including GlucoPro and Cardioton.
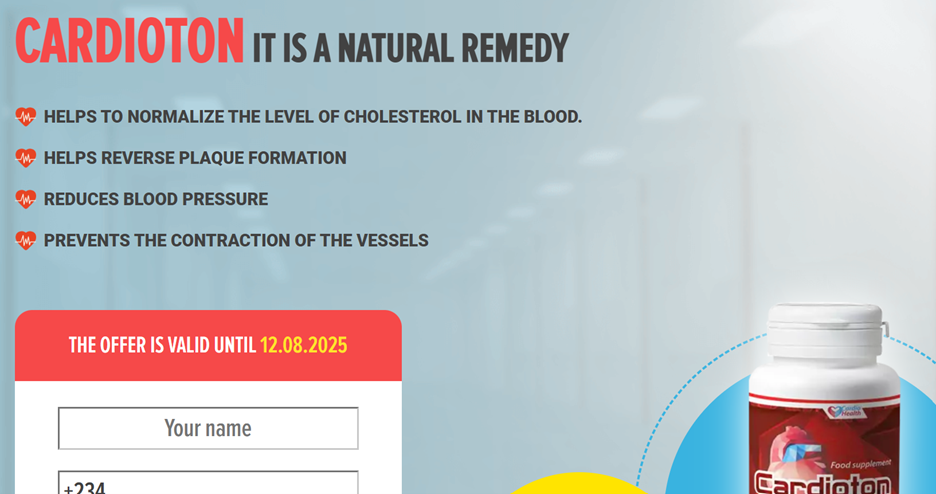
Cardioton online promotion
One of the tools used, DomainTools, revealed that the landing page URLs on the promotions were subdomains to a website, josefinaa.com, which was found to be hosted on Cloudflare.
It was also discovered that Josefinaa.com operated more than 40 subdomains in various languages. These subdomains directed visitors to pages containing articles on health-related topics, many of which promoted and marketed supposed cures for a range of diseases. (Google spreadsheet document to Josefinaa subdomains)
These included having page sections on Josefinaa.com in Swahili, German, Malay, English, Bangla, Hindi, French, and Spanish, all of which were used to promote unlicensed health products marketed as cures for diseases.
They marketed products such as GlucoPro, Normatone, Cardioton, Metabon, DiabeCode, Diabetics, and Fast Active.
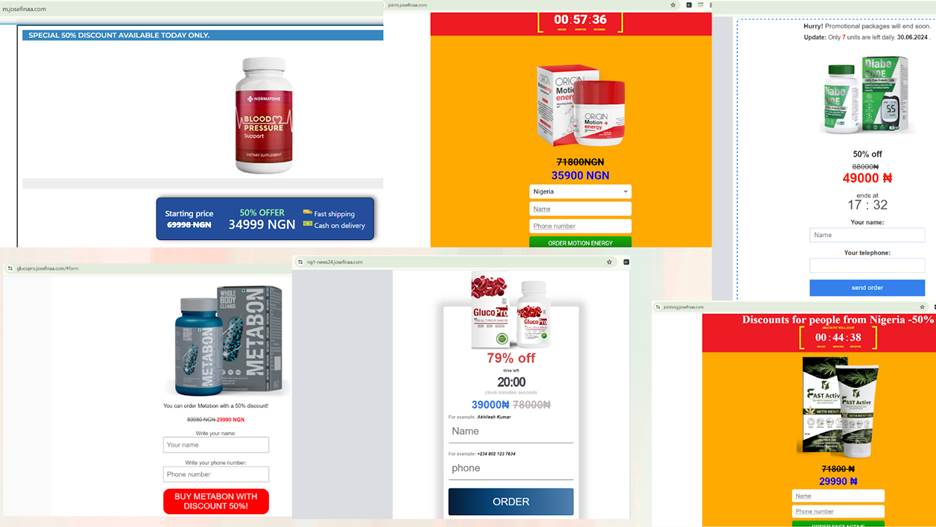
Health products found on Josefinaa.
One of the subdomains had a Channel Television logo and used Bennet Ifeakandu Omalu, a Nigerian-American physician, forensic pathologist, and neuropathologist who was the first to discover and publish findings on chronic traumatic encephalopathy, falsely describing him in a published interview as a cardiovascular surgeon and specialist at the Department of Endocrinology who developed a permanent diabetes cure called GlucoPro. Another subdomain used his images and name to promote a diabetic drug called DiabeCode.
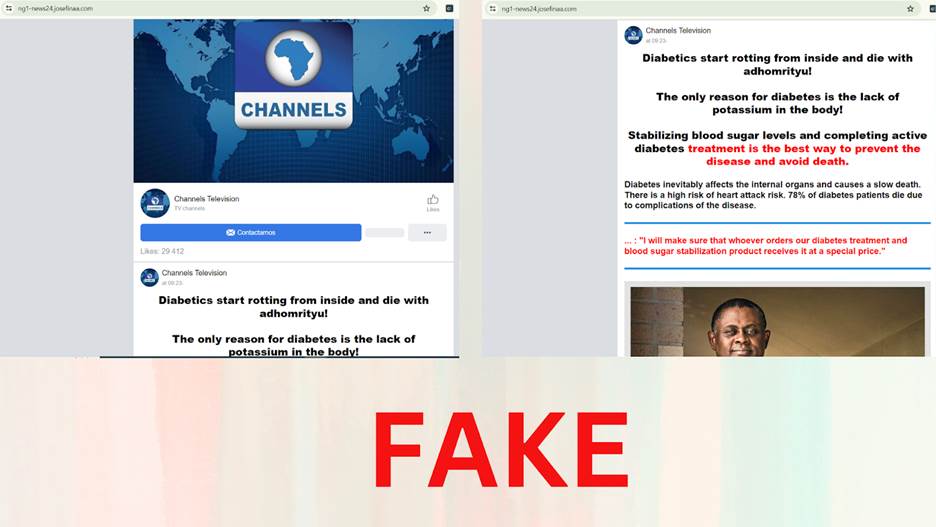
Screenshots from Josefinaa websites using Channels TV logo
The findings were shared with the Post-Marketing Surveillance (PMS) Directorate of the National Agency for Food and Drug Administration and Control (NAFDAC), Fraden Bitrus, and was asked if the government agency had approved the health products promoted on Facebook for sale and use in Nigeria. In response, Bitrus said none had been registered by NAFDAC and therefore should not be on sale.
“Metabon whole body cleanse is under processing for registration but is not registered and should not be on the market for sale,” he said.
Use of a fake location
The information obtained from DomainTools revealed that the website registrant’s name is Aleksandr Kovalenko, the city registrant is in Kiev, and the registrant state or province is Kievskaya.

A comprehensive details on Josefina (Source: DomainTools)
The information alleged that josefinaa.com website was registered in Ukraine, but the information seems suspicious as Kievskaya is a Russian name and does not refer to any city in Ukraine. It is the name of the Moscow Metro station in the Dorogomilovo District, Western Administrative Okrug, Moscow.
One of the Facebook pages monitored, Eco Puzzling, sets its location in Aja, Lagos State, Nigeria, and Laut Products in Delhi, India. However, Facebook uses IP addresses to determine where a page is operating from. Other pages also set their different locations based on what the Facebook page transparency section provided about where the pages are being managed.Facebook shows the location of where the pages are being run from.
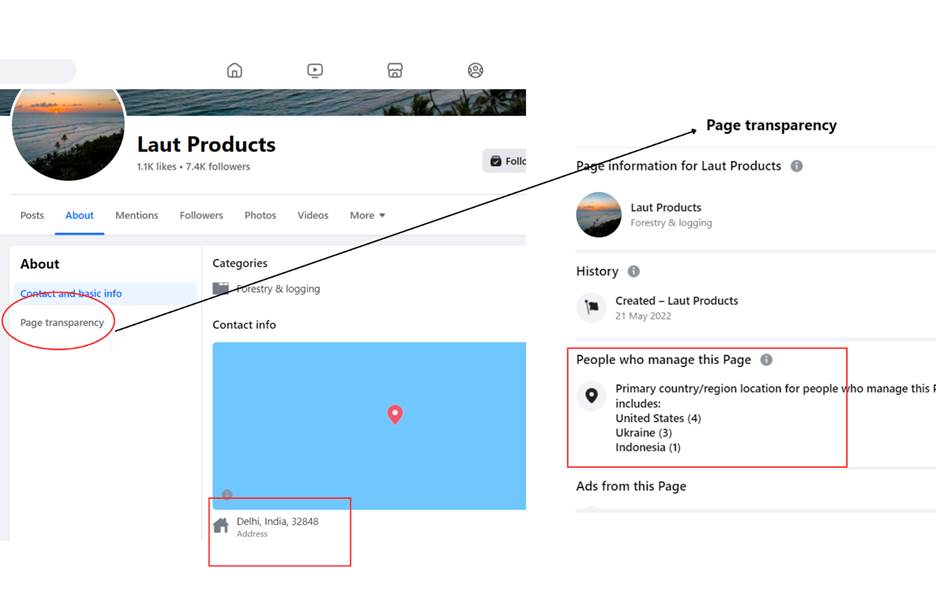
Facebook shows the location of where the pages are being run from.
Using the Facebook transparency feature, the majority of the pages listed administrators’ location in countries such as Ukraine, Nigeria, and the United States; however, some were hidden. Others showed addresses from the Philippines, Indonesia, India, Latvia, Moldova, Russia, Lithuania, and the United Kingdom. The locations, content, promotion, posting patterns and landing pages all point to the same people operating these pages or copying each other.
These pages continue to operate their fraudulent operations and spread misleading health information without being discovered by Facebook, despite the red flags that are visible. Many social media users are suffering and becoming unwitting victims as a result.
In an interview, a legal practitioner, Adedamola Solesi, said Meta cannot be held liable for the videos uploaded on Facebook claiming to have cures for some diseases.
“Meta cannot be held liable for any third-party infringement. Since they have put in mechanisms for any fake news or false news to be reported or flagged, I believe that it is the joint responsibility of not just Facebook alone but also users to report such cases. If we take a cue from the United States, we can see that we have a plethora of court cases vindicating and absolving social media platforms from infringement, crimes, breach of contract, or falsehood that are attributed to third parties,” Solesi said.
However, a human rights activist, Charles Muoneme, disagreed. He said Meta would not absolve itself from the fraud being committed on its platform.
“Meta is liable. If someone perpetrates a crime through a platform you created, you are liable because you have not put sufficient measures in place to curb it. Meta hasn’t done enough to check these deceits on its platform.
Sheu Olayinka is a Data journalist and researcher who specialises in investigating disinformation and digital manipulation. He has worked as a digital and verification expert at The ICIR and The Nation.


